|
Keywords: Keywords: W. Lyman, et l, AIDS, Electric Current,
lymphoblastoid cell lines (H9 and CEM-SS), HIV-1, AIDS, tritiated
thymidine (3H-TdR), HBWSS, Syncytium-formation assay,
varicella, The RF Strain, (SDH), H+(ATP).
BIOCOMPATIBLE ELECTRIC
CURRENT ATTENUATES HIV-I INFECTIVITY
William D. Lyman, Irwin
R.Merkatz
William C. Hatch and Steven C. Kaali
Departments of
Pathology,
and Obstetrics & Gynecology
Albert Einstein. College
of Medicine,
1300 Morris Park Ave., Bronx, N.Y.10461
Running title: Electricity reduces HIV-1
infectivity
Correspondence: Dr, Wm.. D. Lyman
Department of Pathology
Albert
Einstein College of Medicine
1300 Morris Park Avenue
The Bronx, NY
10461
(212) 430-2171
SUMMARY
In this report, we present the results of
double-blinded studies on the use of direct electric current to alter
the infectivity o£ HIV-1 for susceptible cells in vitro.
Two lymphoblastoid cell lines (H9 and CEM-SS) were exposed to aliquots
of the RT strain of HIV-1 treated with direct current. Results of these
studies show that virus treated with currents from 50 to 100
microamperes (ìA) has a significantly reduced infectivity for
susceptible cells.
These experimental
currents were equal to 3.85 and 7.7.ìÁ/mm2 current densities
respectively. The reduction of infectivity was dependent upon, the total
electric charge (ìA x min) passing through the chamber to which the
virus was exposed. Viral infectivity was determined by two independent
measures: a syncytium-formation assay which can be used to quantify the
production of infectious particles; and. a reverse transcriptase assay
which is an index of viral protein production. Additional experiments
demonstrated that the currents employed were biocompatible. Uninfected
H9 cells were exposed to the same conditions used for the viral
aliquots.
There was no significant change
in the percentage of viable uninfected cells exposed to any of the
currents tested. Therefore, because biocompatible direct electric
current attenuates the infectivity of cell-free virus, this treatment
may allow development of new strategies to prevent transmission of HIV-1
through either treating the general blood supply or developing
alternative barrier contraceptive devices. Additionally, biocompatible
electric. current may be applicable for the direct treatment of AIDS
patients by utilizing either extracorporeal systems or self contained
indwelling electrodes. Lastly, because the virus is being attenuated,
electric current may also render treated HIV-1 suitable for vaccine
development.
Key words: HIV-1, AIDS, treatment,
suppression of infectivity, electricity
INTRODUCTION
The number of individuals infected by the human
immunodeficiency virus type-1 (I-(HIV-1) continues to increase on a
world-wide basis (1). A significant percentage, if not all, of these
individuals will eventually develop the acquire d immunodeficiency
syndrome (AIDS) (2)- While horizontal transmission in the homosexual.
population may be contained or decreasing (3), heterosexual transmission
and infection through contaminated blood supplies continues to increase
(4). Additionally ver tical transmission from infected females to their
fetuses is also on the rise with a resultant increase in the number of
children with AIDS (5). New strategies, therefore, must be devised in
order to limit more effectively the spread of this virus.
In this regard, three principal approaches are
currently being investigated. In order to decrease susceptibility to the
consequences of infection, vaccines are being sought which will induce
the production of protective antibodies (6). As treatment modalities,
the use of soluble antagonists to block the receptor for HIV-1 is being
studied (7) as are pharmacologic agents such as nucleic acid analogs
which can interfere with the transcription of viral genomic sequences
(8). Each of these systems has------------ and limitations and to date
none has proven completely effective.
Because heat or light in combination with drugs
and dyes can inactivate viruses including HIV-2 in vitro (9),
others have suggested the use of these forms of energy to treat .. AIDS
patients. The results of studies using heat have not been peer- reviewed
and are therefore impossible to evaluate. The use of light with drugs
["photopheresis"] (10) appears to be efficacious although this treatment
may be limited by drug toxicity and the potential long-term effects of
ultraviolet radiation on blood c ell nucleic acids. Also, by its nature,
this last system may not be suitable for the treatment of
tissue-associated virus.
As result of our interest in the use of
electric current to alter biological systems , we focused our
investigations on the ability of direct electrical current at
biocompatible levels to alter the infectivity of HTV-1 for susceptible
CD4 positive cell s in vitro.
MATERIALS AND
METHODS
Electrical treatment of HIV 1:
The RF strain of HIV-I (AIDS Reagent Program)
was cryopreserved prior to treatment at -70°C. Fur treatment, a sample
of virus was thawed and maintained on ice at 4°C . Ten microliters
(ìl) of HIV-1 at a concentration of 105 infectious particles per ml
were placed into a chamber which included a pair of platinum
electrodes 1mm apart permanently mounted into a well 1.56mm in length
an d 8.32mm in depth equal to 12.9 ìl volume capacity. The chamber was
connected to a power supply capable of creating constant direct
current. The viral aliquots were exposed to direct currents ranging
from 0 microamperes ( ìA) for up to 12 minutes to 100ìA for up to 6
minutes. Intermediate currents of 25, 50 and 75ìA were used to expose
similar viral aliquots. Under these conditions, for example, 0, 50 and
100ìA represent 0, 3.85 and 7 .7ìA/mm2 current densities
respectively. The current was monitored throughout the experiment. A
matrix of current and time employed is shown in Table
1.
After the exposure of virus to electric
current, the contents of the chamber were removed and placed into
sterile microtubes. Five ìl of each sample were removed and diluted
with 95ìl tissue culture medium supplemented with 10% fetal calf serum
(FCS) for subsequent assays.
Syncytium-formation assays:
This assay was performed as previously
described by Nara et al (11). Briefly, 105
CEM-SS cells were dispensed into poly-L-lysine coated microliter
wells. Thereafter, tenfold dilutions o f H9 cells incubated with the
treated HIV-1 samples were co-cultured in triplicate for up to 4 days
with the CEM-SS cells. Identical wells were prepared with control
uninfected and infected cells. The wells were examined for syncytium
formation at 2 and 3 days and quantified using an inverted
microscope.
RReverse trascriptase assay:
Uninfected H9 cells, were pelleted at 1,000
rpm for minutes at room temperature, the supernatant was decanted and
the cells were resuspended in 100ìl treated viral sample. The cells
were incubated for up to 6 hours with the viral samples. At the end of
the incubation time, the viral/cell suspensions were centrifuged at
1,000 RPM for 5 minutes and the supernatant decanted. The cell pellet
was then resuspended in 5ml of RPMI, 10% FCS and placed into a T25
tissue culture flask and maintained at 370C, 5%
CO2 in a humidified chamber. At 2 day intervals (beginning
at day 2}, 1ml of the cell suspensions was removed from each sample
and centrifuged at 1,000 rpm for 5 minutes in order to pellet the
cells. The supernatant was subsequently centrifuged at 14,000 RPM for
I5 minutes. The pellet was resuspended in suspension buffer and
assayed using standard methodology employing Mg+ + as the divalent
cation poly (rA) oligo d(T) 12-18 as template primer, and tritiated
thymidine (3H-TdR) which comprise the reaction mixture.
Known HIV positive and negative control samples were included in each
assay for reference. Thirty ìl of the reacti on mixture were added to
each 10 ìl viral sample and incubated at 37 0C for 60 min.
Samples were then incubated with 1ml of cold quench solution on ice
for 15 minutes and filtered through a Millipore manifold. Chimneys
were rinsed first with wash solution and followed by cold 95% ethanol.
The filters were dried by vacuum and counted in scintillation fluid.
Reverse transcriptase activity is expressed as counts per minute (cpm)
and is considered positive only if cpm are at least five times greater
than the cpm obtained with HIV negative control samples.
Biocompatibility of electric
currents/time:
To determine if the electric currents used
were in a biocompatibility range of energy, uninfected H9 cells were
exposed to distinct currents for different amounts of time. The H9
cells were washed two times in Hanks Balance Salt Solution (HBSS).
Thereafter, the cells were resuspended in RPMI, 10% FCS at a
concentration of 106 cells per ml, Ten ìl of the cell
samples were placed into the reaction c hamber. The cell samples were
then exposed to 0, 50 or 100ìA for 0, 3 or 6 minutes. At the end of
each test, the cell sample was removed from the chamber and
approximately 10ìl of the sample was mixed with 90ìl of trypan blue.
The number of viable cells w as determined by trypan blue exclusion
using a hemocytometer and tight microscope. Results are expressed as
percentage of viable cells from the total of all cells. At least 200
cells per field were counted.
Statistical analysis:
Results of the syncytium-formation and reverse
transcriptase assays were tested for statistical significance by the
Student's t test and analyses of variance.
RESULTS
Syncytium-formation assay:
Using this index of HIV-1 infectivity, it was
determined that exposing virus to direct electric current suppressed
its capacity to induce the formation of syncytia. Figure 1 shows a
representative e xperiment and Table 2 shows the Croup data for 3
separate experiments. As can be noted in Figure l, a statistically
significant (p<0.001) reduction in sycytium number was observed and
this reduction was dependent upon the current applied to the viral i
solate. At three different viral dilutions, there were analogous
results in that a total charge of 200ìA x min (25ìA for 8 minutes)
reduced the number of syncytia from 50 to 65% while a charge of 300ìA
x min (50ìA for 6 minute s, 75ìA for 4 minutes or 100ìA for 3 minutes)
resulted in 90% reduction.
Reverse transcriptase assays:
The direct electric currents to which HIV-1
was exposed also reduced reverse transcriptase activity. Five separate
experiments were conducted and a representative experiment is shown in
Figure 2 and the ;coup data are included in Table 3. As can be seen in
Figure 2, there was a significant decrease in the amount of reverse
transcriptase activity after exposure of the virus to either 50ìA for
3 or 6 minutes. An equivalent reduction in reverse transcriptase
activity was also noted with exposure to, 100ìA for 3 minutes and
almost ablation of reverse transcriptase activity was seen with
exposure of the viral isolate to 100ìA for 6 minutes. The group data
(Table 3} show that after exposure to 50ìA for 6 minutes, there was a
44% reduction in activity and treatment of virus with 100ìA for 6
minutes resulted in a 94% reduction. An analysis of variance indicates
that t he decrease in reverse transcriptase activity was statistically
significant (p <0.0001).
Biocompatibility of the electric
currents/time:
The results of a viability analysis using
trypan blue exclusion criteria applied to uninfected cells exposed to
the different currents and times used far these studies are shown in
Table 4. The viability of H9 cells, after exposure to 100ìA fur either
3 or b minutes, did not show a significant decrease when compared to
the 0 Current control. After maximum treatment at 100ìA for 6 minutes,
cell viability was 93%. Interestingly, in other preliminary
experiments in which HIV-infected H9 cells were used, the results show
that at 100 ìA there may have been a significant decrease in the
number of viable cells. That is, while an insta ntaneous pulse of 100
ìA did not affect the viability of infected cells, at 3 and 6 minutes
of exposure to 100 ìA, a decrease in viability was noted. This
decrease was time dependent in that exposure to 100 ìA far 3 min utes
resulted in a viability of 83% while 100 ìA for 6 minutes resulted in
a viability of 80%. Although these data are provocative, they only
represent a preliminary experiment and require further
investigation.
With respect to the possibility that the
electric current was transduced into heat, the calculated rise in
temperature within the chamber was determined to be less than 1°C. In
order to verify this, a temperature microprobe was introduced into the
cham ber containing tissue culture medium alone. Results of these
studies are shown in Table S. Similar results were obtained when H9
cell-containing medium was placed in the reaction chamber. The data
indicate that for the currents and times used for these ex periments,
there was no alteration in the temperature of the
chamber.
DISCUSSION
The results reported here demonstrate that
HIV-1 treated with direct electric currents from 50 to 100ìA has a
significantly reduced infectivity for susceptible cells in
vitro. This reduction o f infectivity correlates with the total
electric change passing through the chamber. Although extrapolation of
these data predicts that ablation of HIV infectivity may be possible,
and additional preliminary data support this prediction, the expectation
t hat some virions may still escape the electrical effect cannot be
discounted. Nevertheless, the .therapeutic potential of electric current
may reside in its ability to lower the viral titer to subclinical
significance or in its incorporation into a strate gy analogous to that
of other therapies in which repeated cycles of treatment eventually
achieve remission or cure.
The data presented in this report are based on
both quantitative and quantal determinations of viral infectivity.
Although the syncytium-formation assay can be used to quantify the
number of infectious viral particles, this use with respect to HI V-1
may be abridged because of the ability of free fusigenic peptide (gp41)
to induce syncytia by itself. Therefore, while syncytia were observed at
some dilutions of electrically-treated virus, this may simply represent
the presence of soluble gp41 in th e tissue culture medium. We believe
that the correlation between total charge and reduction in syncytium
number more adequately reflects the ability of direct electric current
to reduce HIV-1 infectivity.
This belief is also supported by the results of
the reverse transcriptase assays.
Although a decrease in HIV-1 reverse
transcriptase does not assure reduced infectiousness of this virus for
Susceptible cells; we feel that, taken together with the
syncytium-formation data, the results indicate that significant attenua
tion of HIV-I infectivity is achieved by treatment with direct electric
currents.
With respect to the biocompatibility of the
electric currents and total charges reported here, two separate sets of
evidence are applicable. The first has to do with the results showing
that, by trypan blue exclusion, no significant cyt otoxicity was induced
in by any total charge tested. The other evidence is obtained from
reports which clearly indicates that the amount of electricity used for
these experiments is significantly below presently used therapeutic
electric currents which ar e in the milliampere range (12-16).
Rather than negative effects, exposure of cells
to electric current may actually have positive consequences for
resistance to infection in that important cellular electrochemical
changes correlate with enhancement of specific enzymatic activities. In
particular, a facilitation of succinate dehydrogenase (SDH) and
ATPase activity has been observed (12,15). Both of these enzymes are
associated with the oxidative capacity of the cell. Specifically, it has
been suggested that an elec trochemical reaction occurs between
mitochondrial membrane-bound H+ ATPase and ADP leading to the
formation of ATP. Therefore, exposure of cells to direct electric
current may directly or indirectly increase energy resources within a
cell and facil itate cell metabolism. This, in turn, may actualIy render
a cell less susceptible to the effects of viral infection.
In summary, the data presented here indicate
that biocompatible direct electric current significantly reduces the
infectivity of HIV-1. Continuing investigations are exploring the
mechanisms through which this effect is mediated. The in itial focus of
these experiments is centered on the potential role which ionic and
molecular species generated by electrolysis may have on the virus.
However, the complete mechanism by which direct electric current
attenuates HIV-1 infectivity is undoubte dly far re complex than simple
electrolysis. Nonetheless. and independent of a complete understanding
of all of the mechanisms involved in the attenuation of HIV-1
infectivity, the present observations may serve as an initiaI step for
the development of new strategies to treat infection or prevent
transmission of HIV-1 through either treat ing the general blood supply
or developing alternative barrier contraceptive devices. It may also be
feasible to treat AIDS patients with direct electric current using
either extracorporeal systems or self contained indwelling electrodes.
Lastly, because viral infectivity is being attenuated, electric current
may render treated HIV-1 suitable for vaccine
development.
ACKNOWLEDGMENTS
Thanks go to Mrs. Agnes Geoghan for her
excellent secretarial assistance and to Dr.Gabor, Kemeny for important
technical help. Additional thanks go to Drs. Frank Lilly and Philip
Aisen for their constructive criticism of this
manuscript.
LEGENDS
|
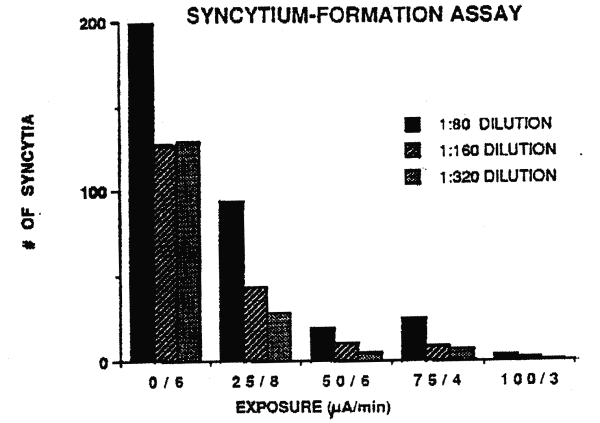
Figure 1. Results of a
representative syncytium-formation assay. Five aliquots of the RF
strain of HIV -1 were exposed to direct electric current for up
to 8 minutes. Three of the samples were exposed to a total electric
charge of 300.ìA x min (50/6, 75/4 and 100/3). At all the dilutions
tested ( shown here), electrical treatment of the virus aliquots
resulted in a significant decrease in syncytium
formation. |
|
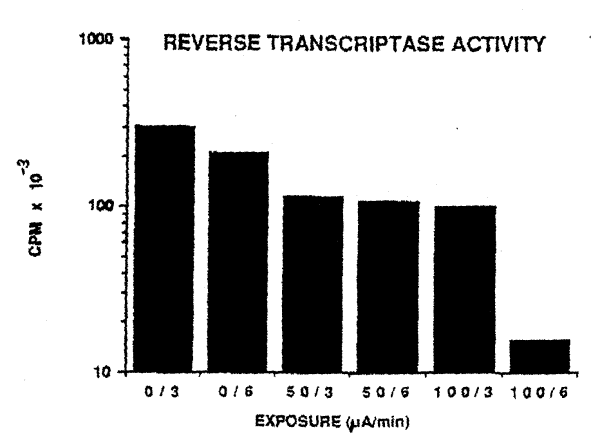
Figure 2. Results of a representative reverse
transcriptase assay. Six aliquots o£ the RF strain of HIIV-1 were
exposed to different amounts .of current for 3 or 6 minutes. A.
significant decrease (p < 0. 005)from 0 current levels (0/3 and
0/6) in reverse transcriptase activity is noted. However, the
decrease is more significant (p<0.0001) when virus is exposed to
100ìA for 6 minutes. |
|
Table
1
Experimental
Paradigm
Current (ì.A). Time (Minutes)
| 0 |
1 4 8 12 |
| 25 |
2 4 8 12 |
| 50 |
3 4 6 12 |
| 75 |
2 4 8 12 |
| 100 |
1 3 4
12 | |
|
Table
2
Effect of ELECTRIC Current on
Syncytium Formationa
% of O Current Control
(Ä%)b
Current (ìA) Six Minute Exposure
| 0 |
100
(0) |
| 50 |
50
(-50) |
| 100 |
35
(-65) |
a = Value at I:160 dilution of virus.
b =
Value equals the mean of 3
experiments. |
|
Table
3
Effect of Electric Current on
Reverse
Transcriptase Activitya
% of O Current
Control (Ä%)
Current (ìa) Six Minute Exposure
| 0 |
100
(0) |
| 50 |
56
(-44) |
| 100 |
6
(-94) |
a = Value equals the mean of 5 experiments.
The standard error of the mean in each case was
less than10% of the mean
value. |
|
Table
4
Effect of Eclectic Current
onViability of Uninfected H9 Cells
(% Viable
CeIIsa)
Length of exposure (Minutes), Current (ìA) 0 3
6
| 0 |
96 94
6 |
| 50 |
98 95
98 |
| 100 |
96 97
93 |
a = At feast 200 cells counted in
hemocytometer field |
|
Table
5
Effect of Electric Current on
Temperature of
Tissue Culture Medium a (°C) Length of
Exposure (Minutes)
| Current (ìA) |
0 3 6 |
| 0 |
19 19 19 |
| 50 |
19 19 19 |
| 100 |
19 19
19 |
a = The temperature was monitored before,
during and after exposure.
Results shown are end-point
determinations. |
REFERENCES
1. Sato PA, Chin J, Mann JM. Review of AIDS and HIV infection Giobal
epidermiology and statistics. AIDS 1989; 3 Suppl.1:S301-7.
2. Centers for Disease Control. Revision of the CDC surveillance case
definition for acquired immunodeficiency syndrome. MMWR 1987; 1 Suppl.
36:S1-15.
3. Thacker SB, Berkelman RL. Public health surveillance in the United
States. Epidemiol. Rev 1988; 10:164.90.
4. Klein RS, Friedland GH. Transmission of human immunodeficiency virus
type (HIV-1) by exposure to blood: Defining the risk. Ann Int Med 1990;
113:729-30.
5. Oxtoby MJ. Epidemiology of pediatric AIDS in the United States. In:
Brain in Pediatric AIDS (Kozlowski PB, Snider DA, Vietze PM, Wisniewski
HM, eds) 1990:1-8
6. Broder S, Mitsuya H, Yarchoan R, Pavlakis GN. Antiretroviral therapy
in AIDS. Ann Int Med 1990: 113:604-18.
7. Perno CF, Baseler MW, Broder S, Yarchoan R. Infection of monocytes
by human immunodeficiency virus I blocked by inhibitors of CD4-gp120
binding, even in the presence of enhancing antibodies. J Exp Med 1990;
I71:1043-56.
8. Mitsuya H, Weinhold KJ, Furman FA et al. 3'-Azido-3'-deoxythymidine
(BW A509U): an antiviral agent that inhibits the infectivity and
cytopathic effect of human T-lymphotropic virus type III/
lymphadenopathy-associated virus in vitro Proc Natl Acad Sci USA 1985;
82:7096-100.
9. Quinnan GV, Wells MA, Wittek AE, et al. Inactivation of human T-cell
virus, type III by heat, chemicals and irradiation. Transfusion 1986;
26:481-3.
10. Bisaccia E, Berger C, KIainer AS. Extracorporeal photopheresis in
the treatment of AIDS-related complex: A pilot study. Ann Int Med 1990;
113:270-75.
11. Nara PL, Hatch WC, Dunlop NM, et al.: Simple, rapid quantitative,
syncytium-forming microassay for the detection of human immunodeficiency
virus neutralizing antibody. Aids Res Hum Retrovirus 1987; 3:283-302
12. Cheng N, Van Hoof H, Bockx E, et al. The effects of electric
currents on ATP generation, protein synthesis, and membrane transport in
rat skin. Clin Ortho ReI Res 1982; 17I:26472.
13. Frank G, Schachar N, Dittrich D, et al. Electromagnetic stimulation
of ligament healing in rabbits. Clin Ortho ReI Res 1983; I75:263-72.
14. Eriksson E, Haggmark T. Comparison of isometric muscle training and
electrical stimulation supplementing isometric muscle training in the
recovery after major knee ligament surgery. Amer J Sports Med 19?9;
7:159-71.
15. Stanish WD, Valiant GA, Bonen A, et al. The effects of
immobilization and of electrical stimulation on muscle glycogen and
myofibrillar ATPase. Can J Appl Sport Sci 1982; 7:267-71.'
16. Pills AA. Electrochemical information transfer at living cell
membranes. Ann NY Acad Sci 1974; 205:148-70.

|


